Which of These Describes the Rate of This Chemical Reaction
The rate of the reaction will increase with increasing concentration of H2O2. If the exponent m is 1 the reaction is first order with respect to A.
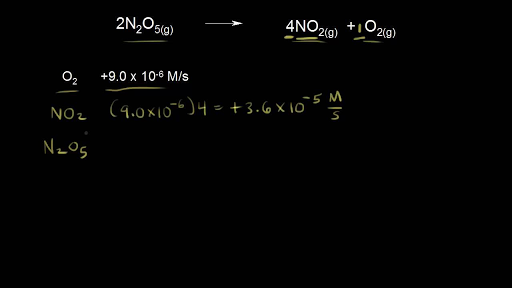
Introduction To Reaction Rates Video Khan Academy
The concentration of the reactants.
. Consider a reaction for which the rate law is. Increasing temperature increases reaction rate. - increase the rate of reaction by adding a substance that helps bring the reactions together so that they can react.
It slows down a chemical reaction. Rate kAmBn rate k A m B n. Gases react more readily than liquids which react more readily than solids.
D add a catalyst. If the reaction is repeated with larger pieces of manganese dioxide which one of the following statements is true. It gives some insight into the time frame under which a reaction can be completed.
The more concentrated the faster the rate. The rate of a reaction is usually observed by watching the disappearance of a reactant or the appearance of a product within a given time period. The amount of a substance in a given volume.
For example the reaction rate of the combustion of cellulose in fire is very high and the reaction is completed in less than a second. Take the chemical reaction. It speeds up a reaction but is not permanently changed.
Reaction rates are therefore determined by measuring the time dependence of some property that can be related to. Investigate factors which affect the speed of a chemical reaction and calculate the time taken for the reaction to occur in National 5 Chemistry. TF An enzyme is a biological catalyst.
Reaction rate is. To be specific it can be expressed in terms of. Which of these describes the rate of this chemical reaction.
TF A catalyst is considered as a reactant in a chemical reaction. More than 250 cm 3 of oxygen gas are produced. Likewise the rate of a chemical reaction is a measure of how much reactant is consumed or how much product is produced by the reaction in a given amount of time.
Some reactions can proceed at explosively fast rates like the detonation of fireworks Figure 171 Fireworks at Night Over River while others can occur at a sluggish rate over many years like the rusting of barbed wire exposed to the. In chemistry the rate of a reaction describes how fast a reaction proceeds over time. - during a chemical reaction there are many particles.
In other words a rate of reaction measures how quickly reactants are changed into products. Chemical Kinetics is the study of reaction rates how reaction rates change under varying conditions and by which mechanism the reaction proceeds. What are the factors that affect the rate of a reaction.
The rate of decrease in concentration of any of the reactants. A usually is increased when the concentration of one of the reactants is increased B is dependent on temperature C may be increased by certain catalytic agents D will be very rapid if the activation energy is large E describes the change in concentration of a. Reaction kinetics is the study of the rate of chemical reactions and reaction rates can vary greatly over a large range of time scales.
Effect of Temperature on Reaction Rate 1. The rate of reaction is the change in the amount of a reactant or product per unit time. Inhibitors increase the rate of a reaction.
The rate of reaction for the decomposition of hydrogen peroxide to yield water and oxygen is given by the equation. The rate of a chemical reaction. A2B 3C A 2 B 3 C.
Define the rate of a chemical reaction. Pour about 5 mL of 60 M HCl into each of three clean test tubes. The rate of increase in the concentration of any of the products.
Place one of the tubes in. Some chemical reactions are neither slow nor fast but take place at a moderate rate. The minimum amount of energy needed to start any reaction.
The rate of the reaction is unaffected by the concentration of H2O2. How do you compare rate with the equilibrium of a chemical reaction. Describe what catalase is and does - help to quickly breakdown hydrogen peroxide which is produced in cells as a toxic byproducts of normal cellular process.
Grinding a seltzer tablet into powder increases the rate of reaction due to increased. The reactants are made up of gaseous particles for gases reacting the rate of the reaction is directly proportional to concentration. Choose the INCORRECT answer.
TF The speed of a reaction can be increased by increasing reactant concentration or decreasing particle size. Here the rate of appearance of product C in time interval Δt is. Increasing this speeds up most chemical reactions.
In a solution increasing the amount of reactants increases the reaction rate. Catalysts on the rates of chemical reactions and then explain these effects in terms of the collision theory. The speed or rate of a chemical reaction is the change in concentration of a reactant or product per unit time.
There are five general properties that can affect the rate of a reaction. The exponents in a rate law describe the effects of the reactant concentrations on the reaction rate and define the reaction order. See the answer See the answer See the answer done loading.
When powdered manganese dioxide is added to a hydrogen peroxide solution 250 cm 3 of oxygen gas are produced in 3 minutes. This helps to speed up a reaction but does not take part in the chemical reaction. The exponents in a rate-law expression define the _____ which describe how the rate is affected by the concentration of the rectant REACTION ORDER The following theory is the theory that states that atom molecules ions must collide for a chemical reaction to occur.
The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. H2 Cl2 2 HCl. Average rate ΔC Δt average rate Δ C Δ t.
This problem has been solved. Increasing pressure increases reaction rate. If m is 2 the reaction is second order with respect to A.
Less than 250 cm 3 of oxygen gas are produced. In an endothermic reaction heat is Q. A catalyst lowers activation energy increasing reaction rate.
Which of the following is indicated by this equation. Factors that affect the rate of a reaction. For this kind of chemical reaction the rate would increase if the concentration of the reactants increase.
What Are The Units Of Rate Of Reaction In Chemistry Quora
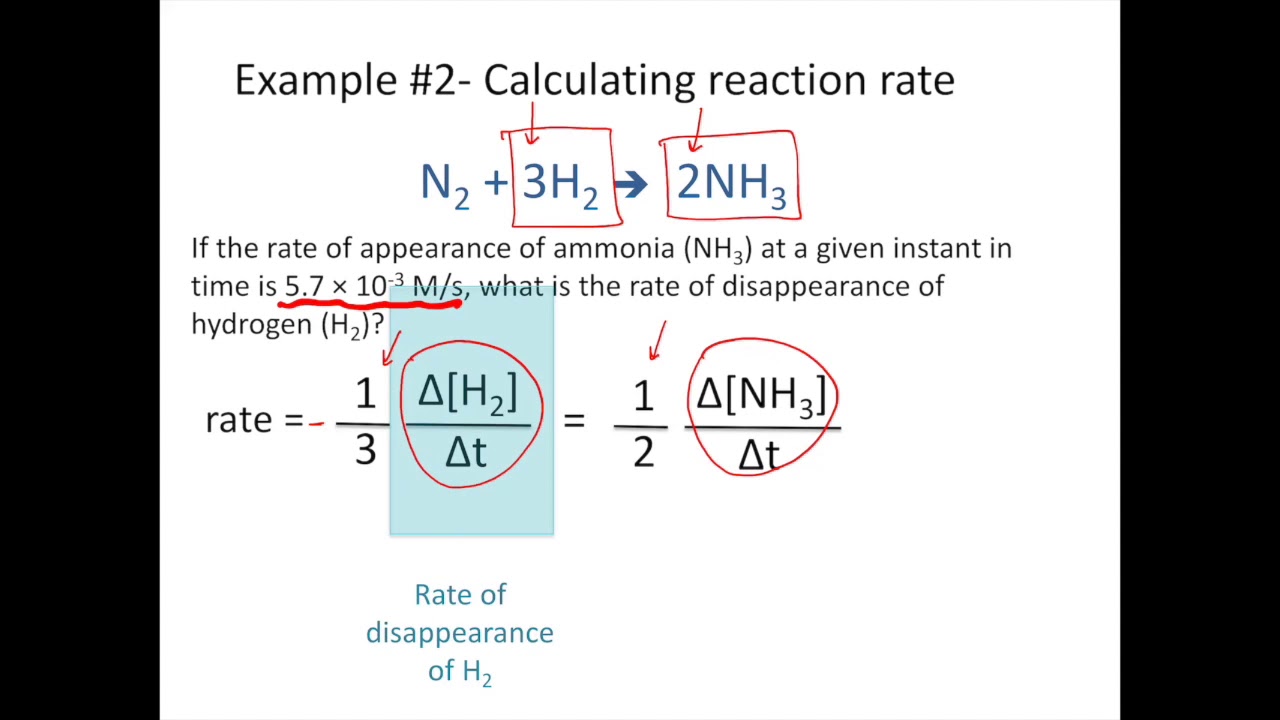
Chemical Reaction Rates Chemistry For Majors

Metabolism Is A Term That Describes All The Chemical Reactions In Your Body These Chemical Reactions Keep Your High Metabolism Metabolism Booster Metabolism
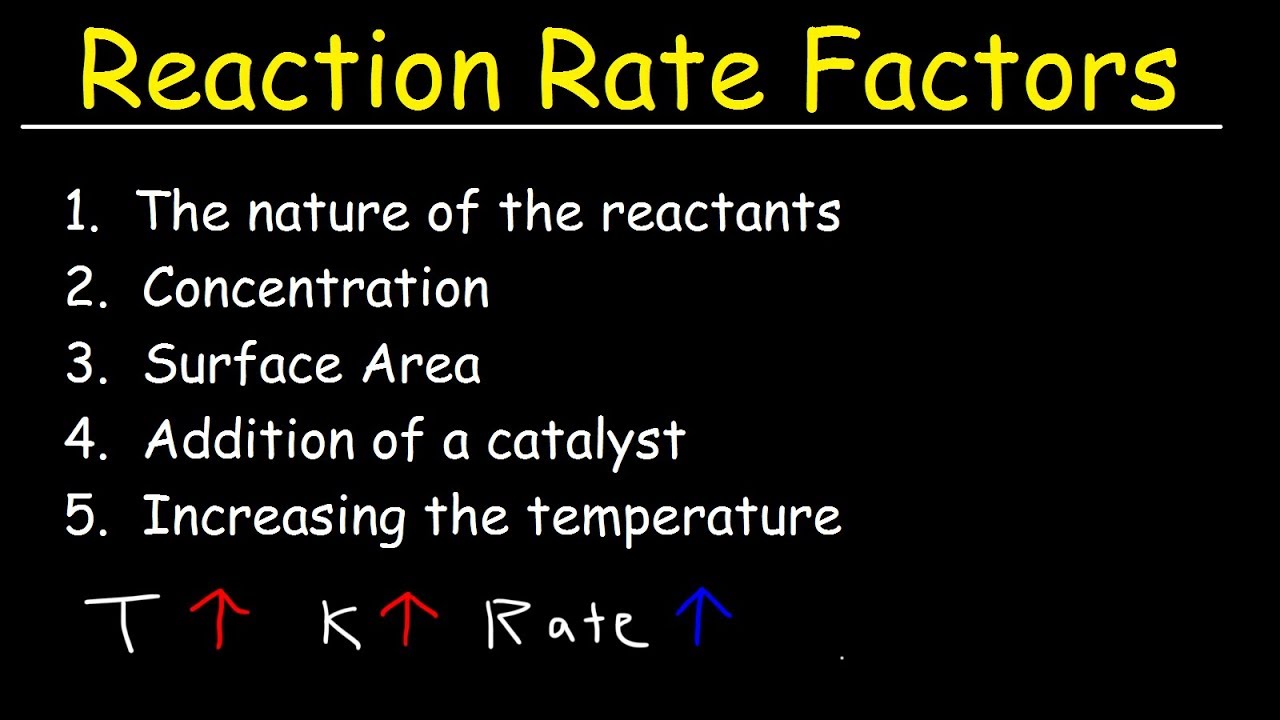
Factors Affecting The Rate Of The Reaction Chemical Kinetics Youtube
No comments for "Which of These Describes the Rate of This Chemical Reaction"
Post a Comment